Rimkus has acquired Human Factors Consulting Services, Inc. Learn more here.
Human Factors Behavioral Research & Design
"Small Team Agility, Large Company Quality"

OUR SERVICES
HFCSI provides a wide range of verification and validation services including (but not limited to):
- Gap Analysis
- Expert Heuristic Analysis
- Formative Testing
- Summative / Validation Testing
- Actual Use Testing
- IFU Development and Testing
- Prototype Testing and Down-Selection
- Research Specialization w/Spanish Speaking Populations
- Marketing Research and HF Testing Integrated!
- Post-Market Evaluation
- Ethnographic Research
- Other needs? Contact us!
HFCSI has joined the Rimkus Family of Companies as part of the Core Human Factors group. Learn more about the human factors analysis services Core can provide through research and guidance on consumer, industrial, and healthcare-related products here.
Gap Analysis
Evaluation of your product’s current design and documentation, and identification of any missing components needed to meet FDA and International standards. HFCSI can then specify a complete road map for providing all HF requirements needed for a successful submission.
Expert Heuristic Analysis
This is a fast, inexpensive way to determine major design issues before performing costly studies. HFCSI uses a mix of standard and proprietary techniques to produce this precision snapshot summarizing issues likely to result in critical use errors or significant usability problems.
Formative Testing
Exploratory and targeted studies to guide development and prepare you for validation. HFCSI’s capabilities include any testing needed, from early market explorations to pre-Summative “dress rehearsals”. We specialize in providing you with design-relevant data presentations.
Summative and Actual Use Testing
Comprehensive validation testing provides evidence for submission to the FDA and EU. HFCSI can handle all aspects of test design, recruitment, data collection, statistical testing and root cause analyses.
IFU Development and Testing
IFUs (Instructions for Use) are crucial for successful products & FDA submissions. Are your IFUs designed at the end of development? Is your IFU production not user-centric (most are not!)? Are you experiencing a delayed approval due to an ineffective IFU? If any of these questions sound familiar, contact us to discuss our Task-Driven IFU® devlopment and testing service.
Prototype Testing and Down-Selection
Customized testing to evaluate the relative performance and/or preference of alternative designs your team is considering. Provides invaluable insights to guide early development.
Research Specialization with Spanish-Speaking Populations
Marketing Research and HF Testing Integrated!
HFCSI can perform early market studies or post-launch evaluations. We also offer unique combinations of marketing-oriented research with usability studies or human factors heuristic or performance studies. A blend of our techniques can provide important cross-functional insights.
Post-Market Evaluation
Gathering intelligence on how your product is performing in the field provides insights to guide future development. Methodologies are customized for each client depending on your specific objectives (e.g., to address known pain points, conduct general assessments of user
groups, gather subjective insights, compare international to domestic markets, etc.)
Ethnographic Research
We can test completed products or advanced product prototypes in the field where users would use, or are currently using, your product. Both end users and/or HCPs can be studied.
…Or do you have “Other” usability or UX needs? HFCSI can provide other related analyses and customized services. Feel free to contact us to discuss your project.
OUR EXPERIENCE
Since our inception in 2006, HFCSI has:
- Worked on a diverse array of medical products for 50+ clients, across a variety of disease states and user populations
- Conducted approximately 300 explorative and formative studies and 75 summative validation studies
- Had no FDA rejections of our HF validation studies since our founding
Recent Projects
Pen injectors, Autoinjectors,
& On-Body Injectors
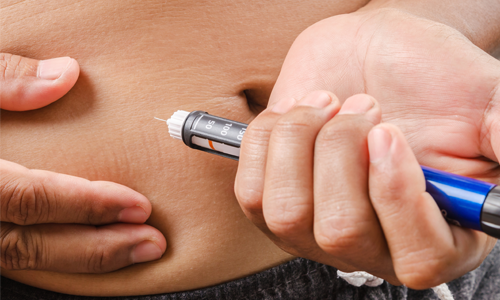
Combination Products; multiple device formats for therapies including rare conditions (e.g. hemophilia, HGH, PAH, etc.)
Digital Health Apps
& Mobile Connectivity

Mobile app development integrated with state of the art devices, legacy devices, enterprise systems and cloud-based data analysis
Glucose Sensors
(CGM)

UX requirements, complete UI design, prototype down-selection, life-cycle HF research
Mechanical
Ventilators
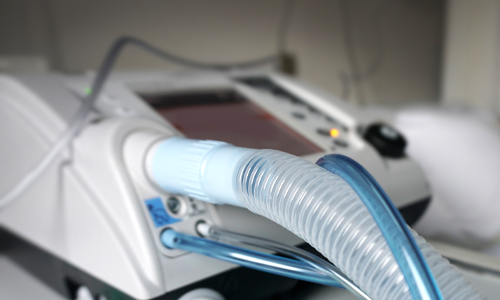
Validation for updates to legacy devices and next generation touchscreen ventilators
Hemodialysis
Machines
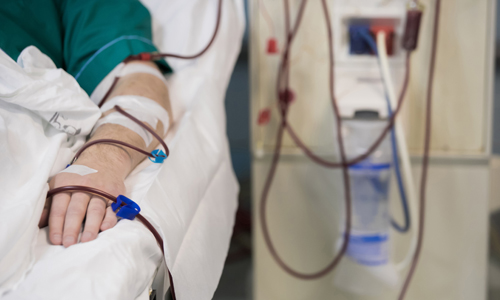
Formative and Summative testing for regulatory filing
Hospital Monitoring
Devices

System Design: from user requirements through design verification
Smart Infusion Pumps
for Hospital Use

Requirements definition, UI development, controllers and durables, V&V testing
HCP Informatics
& Dynamic Displays

System design: from concept development through final validation testing
Recent User Populations
Anemia
Ankylosing Spondylitis
Cancer and chemo complications
Cardiovascular Diseases
COPD
Crohn’s Disease
Diabetes
Glaucoma
High Cholesterol
ITP
Juvenile Idiopathic Arthritis
Kidney Disease (Dialysis)
Migraine
Neurological conditions
Nonalcoholic Fatty Liver Disease
Noonan Syndrome
Osteoporosis
Plaque Psoriasis
Psoriatic Arthritis
Pulmonary Arterial Hypertension
Respiratory conditions
Rheumatoid Arthritis
SGA
Turner Syndrome
Ulcerative Colitis
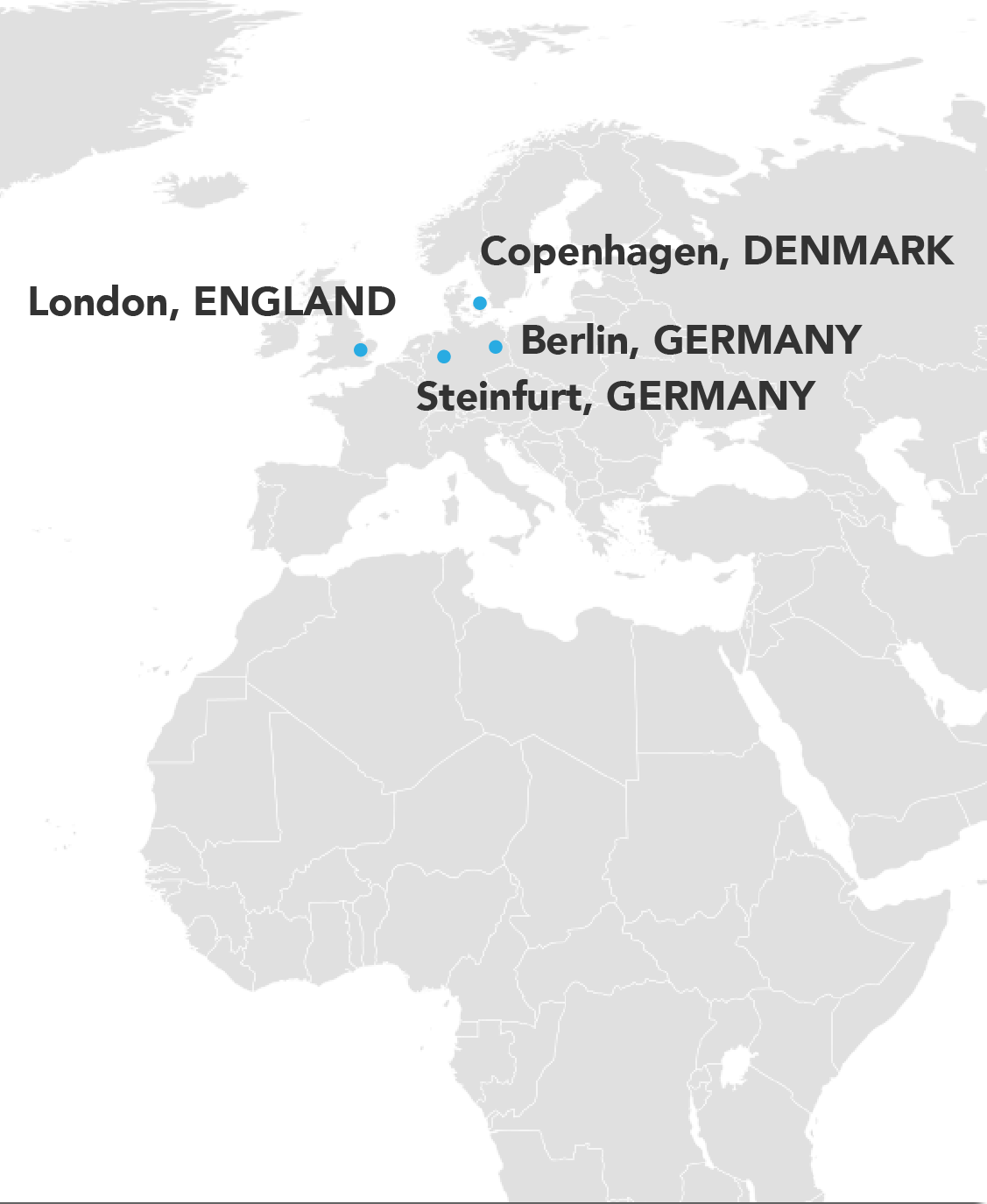
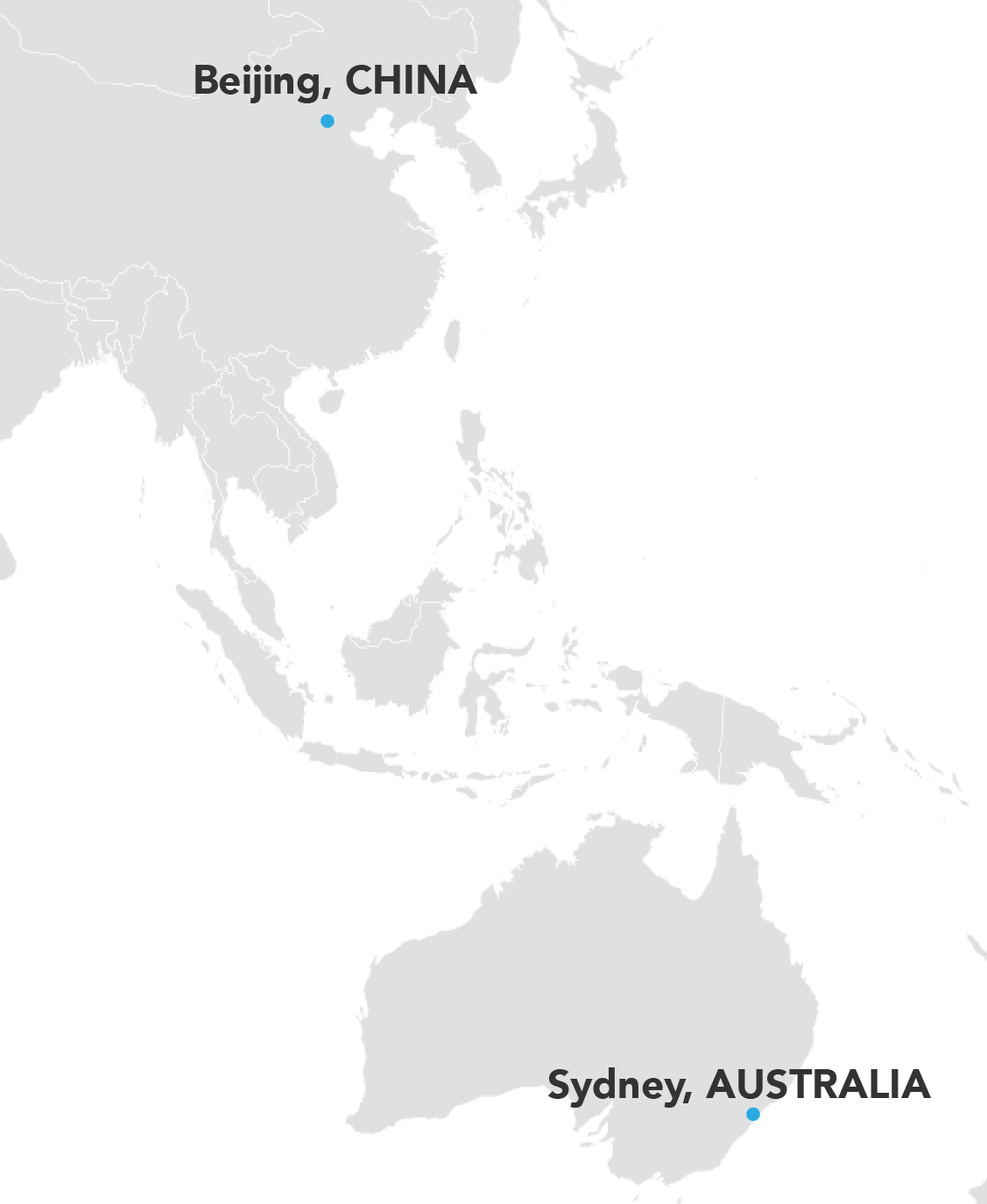
Where We've Worked
We go to the user population! Our projects have taken us all over the world; across the United States, throughout Europe, as well as Asia and Australia.

ABOUT US
What you should know about our company:
- HFCSI was founded in 2006 in Los Angeles, California and operates in the U.S., Europe, Asia, and Australia
- Our team members have Graduate level training in HF psychology, engineering and advanced research methods
- To date, we have conducted usability testing with approximately 5,000 users world-wide…and counting!
- We cover HF/Usability needs in all phases of medical product development
- Our specialists can integrate seamlessly with your cross functional teams
- HFCSI adapts to tightening deadlines or changing requirements
- Our team maintains high standards of quality, service and on-time delivery
- We have a proven 15+ year track record of successful performance
Our Core Team

Mike Kasamanian
Director, Human Factors Practice
Mike Kasamanian
Director, Human Factors Practice
 Mike Kasamanian serves as Director, Human Factors Practice at HFCSI, A Core Human Factors and Rimkus Company, providing expertise in the areas of Human Factors and Technology application. He holds a M.A. degree in Human Factors Applied Psychology and is a certified Quality Function Deployment (QFD) Green Belt. He is an active member of the Human Factors and Ergonomics Society (HFES) and the Association for the Advancement of Medical Instrumentation (AAMI). Mike's expertise includes the following domains: Voice-of-Customer research, User Requirements, Wireframing, Hardware User Interface Design, Rapid Prototyping of Electromechanical devices, FDA verification/validation testing, K-12 instruction, Field Robotics, Multimedia Systems, Acoustic Analysis, and project management.
Mike Kasamanian serves as Director, Human Factors Practice at HFCSI, A Core Human Factors and Rimkus Company, providing expertise in the areas of Human Factors and Technology application. He holds a M.A. degree in Human Factors Applied Psychology and is a certified Quality Function Deployment (QFD) Green Belt. He is an active member of the Human Factors and Ergonomics Society (HFES) and the Association for the Advancement of Medical Instrumentation (AAMI). Mike's expertise includes the following domains: Voice-of-Customer research, User Requirements, Wireframing, Hardware User Interface Design, Rapid Prototyping of Electromechanical devices, FDA verification/validation testing, K-12 instruction, Field Robotics, Multimedia Systems, Acoustic Analysis, and project management.
Zachary Chesler
Director, Human Factors Practice
Zachary Chesler
Director, Human Factors Practice
 Zachary Chesler serves as Director, Human Factors Practice at HFCSI, A Core Human Factors and Rimkus Company, and has over fifteen years experience as a user experience designer and usability researcher. As a specialist in this field, he has worked on projects across several industries including medical/healthcare, training/education, automotive, entertainment, energy management, and digital publishing. Since 2010, Zachary's primary focus has been in the healthcare space, with a specialization in the design and evaluation of medical devices, software, and mobile apps. Zachary earned his Bachelor's degree from University of California, Santa Barbara and a Master's Degree in Human Factors Applied Psychology from California State University, Northridge.
Zachary Chesler serves as Director, Human Factors Practice at HFCSI, A Core Human Factors and Rimkus Company, and has over fifteen years experience as a user experience designer and usability researcher. As a specialist in this field, he has worked on projects across several industries including medical/healthcare, training/education, automotive, entertainment, energy management, and digital publishing. Since 2010, Zachary's primary focus has been in the healthcare space, with a specialization in the design and evaluation of medical devices, software, and mobile apps. Zachary earned his Bachelor's degree from University of California, Santa Barbara and a Master's Degree in Human Factors Applied Psychology from California State University, Northridge.
Wei-Ting Xie
Human Factors Practice Leader
Wei-Ting Xie
Human Factors Practice Leader
 Wei-Ting Xie is a Human Factors Practice Leader at HFCSI, A Core Human Factors and Rimkus Company, with more than 10 years of experience in medical Human Factors. She has expertise in leading and designing human factors research, conducting human factors analyses, developing user requirements, and wireframing / prototyping. Wei-Ting holds a master’s degree in Human Factors and Applied Psychology from California State University, Northridge. In her spare time, she enjoys hiking and is an avid crafter who loves all projects “DIY."
Wei-Ting Xie is a Human Factors Practice Leader at HFCSI, A Core Human Factors and Rimkus Company, with more than 10 years of experience in medical Human Factors. She has expertise in leading and designing human factors research, conducting human factors analyses, developing user requirements, and wireframing / prototyping. Wei-Ting holds a master’s degree in Human Factors and Applied Psychology from California State University, Northridge. In her spare time, she enjoys hiking and is an avid crafter who loves all projects “DIY."
Leah Gudiel
Principal Human Factors Consultant
Leah Gudiel
Principal Human Factors Consultant
 Leah Gudiel is a Principal Human Factors Consultant with experience in software usability evaluations, concept sketches, flow charts, task analysis and usability testing. She holds a Bachelor's Degree in Graphic Design and a Master's Degree in Human Factors & Applied Psychology. Her background in design helps in optimizing prototypes in the creative process to receive the best feedback from potential customers. She enjoys attending music festivals, wine tastings, and exploring faraway countries.
Leah Gudiel is a Principal Human Factors Consultant with experience in software usability evaluations, concept sketches, flow charts, task analysis and usability testing. She holds a Bachelor's Degree in Graphic Design and a Master's Degree in Human Factors & Applied Psychology. Her background in design helps in optimizing prototypes in the creative process to receive the best feedback from potential customers. She enjoys attending music festivals, wine tastings, and exploring faraway countries.
Veronica Mendez
Principal Human Factors Consultant
Veronica Mendez
Principal Human Factors Consultant
 Veronica Mendez is a Principal Human Factors Consultant with a demonstrated history in working with the design of university websites, various application software and medical devices. She is skilled in wireframing, persona development, user needs requirements, protocol development, usability testing (both formative and summative), data analysis and report writing. Veronica graduated cum laude from California State University, Northridge with a Bachelor of Arts Degree in Psychology (minor in Ethnic Studies). Veronica went on to earn a Master of Arts Degree in Human Factors and Applied Psychology from California State University, Northridge.
Veronica Mendez is a Principal Human Factors Consultant with a demonstrated history in working with the design of university websites, various application software and medical devices. She is skilled in wireframing, persona development, user needs requirements, protocol development, usability testing (both formative and summative), data analysis and report writing. Veronica graduated cum laude from California State University, Northridge with a Bachelor of Arts Degree in Psychology (minor in Ethnic Studies). Veronica went on to earn a Master of Arts Degree in Human Factors and Applied Psychology from California State University, Northridge.
Isis Chong
Principal Human Factors Consultant
Isis Chong
Principal Human Factors Consultant
 Isis Chong is a Principal Human Factors Consultant with more than a decade of experience in human factors and experimental psychology. Isis is experienced with applying human factors principles to the design, evaluation, and validation of various medical devices. Isis holds a PhD in Cognitive Psychology from Purdue University and a MS in Human Factors Psychology from California State University, Long Beach.
Isis Chong is a Principal Human Factors Consultant with more than a decade of experience in human factors and experimental psychology. Isis is experienced with applying human factors principles to the design, evaluation, and validation of various medical devices. Isis holds a PhD in Cognitive Psychology from Purdue University and a MS in Human Factors Psychology from California State University, Long Beach.
Vanui Kajberuni
Senior Human Factors Consultant
Vanui Kajberuni
Senior Human Factors Consultant
 Vanui Kajberuni is a Senior Human Factors Consultant who was trained to the Master's level in Human Factors at California State University, Long Beach. She has a BA in Psychology from California State University, Northridge, and an AA in Aviation Science and Commercial Flight. She loves cognitive psychology, attention, and perception. Vanui is a private pilot.
Vanui Kajberuni is a Senior Human Factors Consultant who was trained to the Master's level in Human Factors at California State University, Long Beach. She has a BA in Psychology from California State University, Northridge, and an AA in Aviation Science and Commercial Flight. She loves cognitive psychology, attention, and perception. Vanui is a private pilot.
Yair Villanueva
Human Factors Consultant
Yair Villanueva
Human Factors Consultant
 Yair is a Human Factors Consultant at HFCSI, A Core Human Factors and Rimkus Company. He received his Bachelor’s Degree in Psychology from California State University, Northridge, and is finalizing his Master’s Degree in Service Design from Politecnico di Milano in Italy. His multidisciplinary background allows him to adapt his skillset to the customer and task at hand. He loves traveling, trying new restaurants, and card games.
Yair is a Human Factors Consultant at HFCSI, A Core Human Factors and Rimkus Company. He received his Bachelor’s Degree in Psychology from California State University, Northridge, and is finalizing his Master’s Degree in Service Design from Politecnico di Milano in Italy. His multidisciplinary background allows him to adapt his skillset to the customer and task at hand. He loves traveling, trying new restaurants, and card games.
David Hermoza
Human Factors Consultant
David Hermoza
Human Factors Consultant
 David Hermoza is a Human Factors Consultant with experience in Medical Human Factors. He is a member of the Human Factors and Ergonomics Society (HFES). David earned his Bachelor’s degree in Cognitive Science from University of California, San Diego and is currently completing a Master’s degree in Human Factors from California State University, Long Beach. David focuses on FDA-guided formative and summative Human Factors evaluations, as well as Human-Computer Interaction principles, especially Trust in Automation theories. In his spare time, he likes sketching, watching movies at the movie theater, and visiting National Parks.
David Hermoza is a Human Factors Consultant with experience in Medical Human Factors. He is a member of the Human Factors and Ergonomics Society (HFES). David earned his Bachelor’s degree in Cognitive Science from University of California, San Diego and is currently completing a Master’s degree in Human Factors from California State University, Long Beach. David focuses on FDA-guided formative and summative Human Factors evaluations, as well as Human-Computer Interaction principles, especially Trust in Automation theories. In his spare time, he likes sketching, watching movies at the movie theater, and visiting National Parks.
Arturo Perez
Human Factors Consultant
Arturo Perez
Human Factors Consultant
 Arturo Perez is a Human Factors Consultant with more than 4 years of research experience. Arturo’s journey into human factors began with B2C sales. He became enthralled by the way products meet users’ needs. Arturo then realized that he needed to be an advocate for users. Thus, he earned his BA in Psychology (Minor in Marketing), and is studying towards his MS in Human Factors at the University of Idaho. Arturo’s background in B2C sales, psychology, and research qualify him to put users first.
Arturo Perez is a Human Factors Consultant with more than 4 years of research experience. Arturo’s journey into human factors began with B2C sales. He became enthralled by the way products meet users’ needs. Arturo then realized that he needed to be an advocate for users. Thus, he earned his BA in Psychology (Minor in Marketing), and is studying towards his MS in Human Factors at the University of Idaho. Arturo’s background in B2C sales, psychology, and research qualify him to put users first.
Winnie
Team Mascot
Winnie
Team Mascot
 Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat.
Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat. Loves to eat.
You?
Human Factors Jedi
You?
Human Factors Jedi
 If you are interested in joining our team at HFCSI, please send a message to info@hfcsi.com and tell us a little about yourself!
If you are interested in joining our team at HFCSI, please send a message to info@hfcsi.com and tell us a little about yourself!
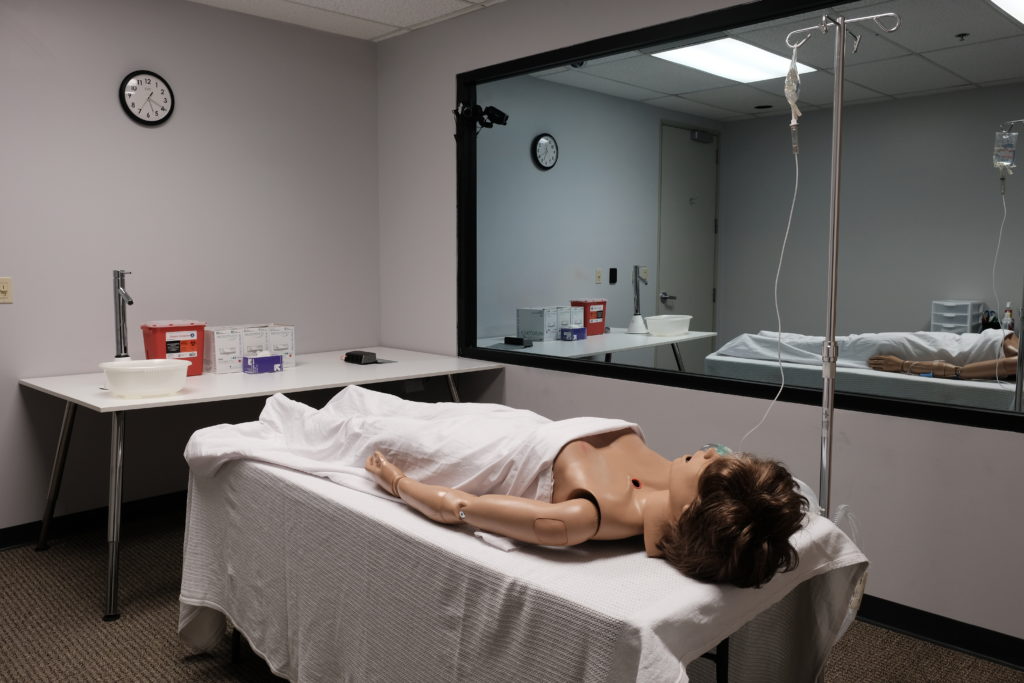
Our Research Facility
Though we have traveled the globe to conduct research with diverse user populations, we also have our own research facility space at our headquarters in Los Angeles.


Professional Affiliations
We are active, contributing members of the following industry organizations, including the authoring of best practice documents/standards, and teaching HF training courses.
Shoot us an email at info@hfcsi.com to ask about our 3-day Usability Testing workshops for Medical Devices with Marcus Evans; Learn the latest best practices to plan studies, stay compliant with agency expectations, and convert data to actionable insights. Get the inside scoop on ways to efficiently scale the level of Human Factors effort and demonstrate due-diligence.
© 2025 Human Factors Consulting Services, Inc.




